International
International
Enabling rapid diagnostic testing in low-resource settings
Enabling upscale of diagnostic manufacture for the COVID-19 pandemic
A pioneer in the development of rapid diagnostic tests, Mologic, a UK-based diagnostic-technologies expert, is a social enterprise with a simple mission – to accelerate access to innovative technologies and products for epidemics and neglected diseases for all nations.
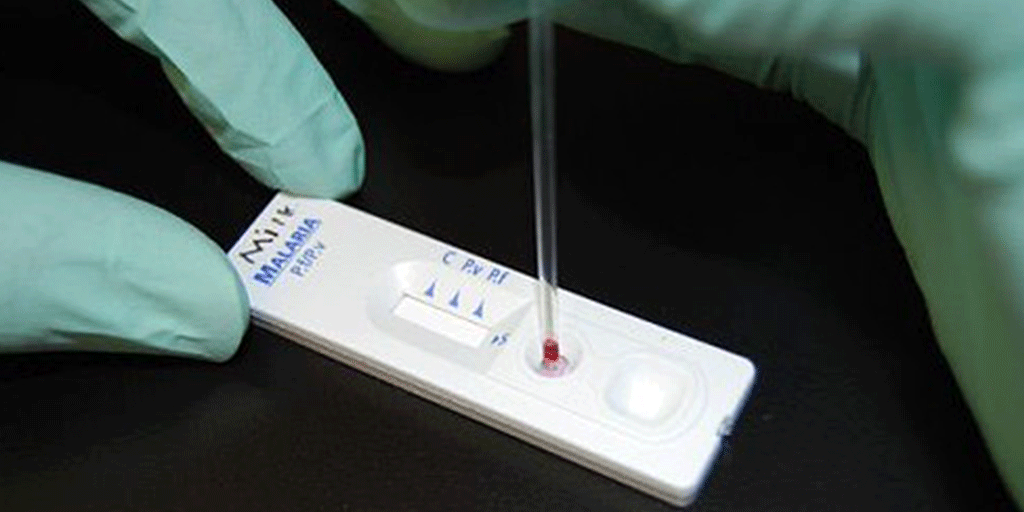
Weeks before the World Health Organisation declared COVID-19 a global pandemic, the race to launch a low-cost rapid diagnostic test (RDT) was already underway. Mologic was refining an RDT that could detect COVID-19 antibodies in ten minutes and needed a way to rapidly scale production for global markets, especially those with limited healthcare support. In parallel, Mologic also launched a further programme to develop a rapid antigen test to detect active infection in respiratory samples.
As a first step, Mologic wanted to scale production in the UK and secure a supply chain for product development and distribution to low-income settings. But it saw an opportunity to do so much more: to foster self-sufficiency at a time of great uncertainty through distributed production – jointly in Europe and Africa.
At that time, with a focus on partnering with other companies it had no ability to scale production beyond 4 million tests a year, and almost no go-to-market infrastructure. Widespread supply chain disruption and building a distribution network to get the test to those who needed it presented additional challenges. Furthermore, it also needed guidance and support in developing a process and manufacturing facility that could be deployed in Africa.
At a time when collaboration and shared purpose were more important than ever, Deloitte recognised that providing support to this Bedford-based SME to bring this test to market quickly could make a real difference to outcomes in international markets without advanced health care infrastructure.
Drawing on expertise in programme management, operations transformation, finance, manufacturing and supply chain, the Deloitte team leveraged its global network to support Mologic in meeting its objectives.
Working mostly pro-bono, Deloitte supported Mologic to bring its COVID-19 test to market four weeks earlier than originally planned and expand production by 1,000%.
The Deloitte team also supported the financial planning for a manufacturing facility in Senegal. The facility supports the development of skills locally. At the same time, it has also built sustainable critical capacity in Africa – vital for the continent’s response to the pandemic and future events and offering security of supply for the region.
This first product laid the groundwork for the Mologic rapid antigen test to quickly follow. Mologic’s RDT antigen test has excellent performance in FIND’s independent evaluations, with >90% sensitivity and 100% specificity, and is a market leader. The greatest benefit of Mologic’s RDT will be felt in resource-limited settings where it will enable nations to upscale testing to understand local epidemiology and direct interventions. Deloitte is proud to have been able to play a part in making this possible.
View Deloitte’s profile in the MCA Members Directory.